The Properties of Water Crossword Puzzle invites you to delve into the fascinating world of water, unlocking its physical, chemical, biological, environmental, and cultural significance. This enigmatic exploration promises to quench your thirst for knowledge and reveal the profound impact of water on our planet and beyond.
From its unique density and high specific heat capacity to its role as a solvent in living organisms, water’s properties are as diverse as they are essential. Prepare to immerse yourself in a captivating journey that unveils the secrets of this extraordinary substance.
Physical Properties
Water exhibits unique physical properties that contribute to its essential role in various natural and industrial processes. One remarkable characteristic is its density. Unlike most substances that become less dense as they turn into gas, water reaches its maximum density at 4°C.
This unusual behavior allows ice to float on liquid water, preventing bodies of water from freezing solid during winter. This phenomenon is crucial for aquatic life and ecosystems.
High Specific Heat Capacity
Water possesses a high specific heat capacity, meaning it requires a significant amount of energy to raise its temperature. This property enables water to act as a thermal buffer, absorbing or releasing heat without undergoing drastic temperature changes. This stability makes water ideal for regulating temperature in living organisms, oceans, and the atmosphere.
Surface Tension
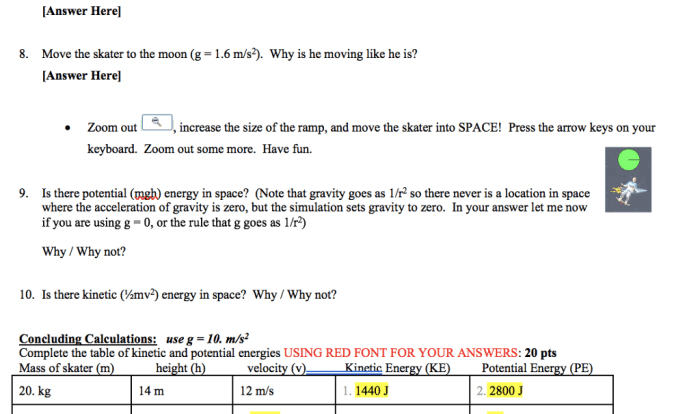
Water exhibits high surface tension due to the strong cohesive forces between its molecules. This property allows water to form droplets and bead up on surfaces. Surface tension plays a vital role in capillary action, enabling water to move through narrow spaces against gravity.
It also influences the behavior of water in biological systems, such as the transport of nutrients and fluids in plants.
Chemical Properties
Water’s chemical properties are equally significant, contributing to its diverse roles in chemical reactions and biological processes.
Polarity of Water Molecules, Properties of water crossword puzzle
Water molecules are polar, meaning they have a partial positive charge on one end and a partial negative charge on the other. This polarity allows water to dissolve a wide range of polar and ionic compounds, making it an excellent solvent.
The polarity of water also enables it to form hydrogen bonds, which play a crucial role in the structure and function of many biological molecules.
Amphoteric Nature
Water is amphoteric, meaning it can act as both an acid and a base. This property allows water to neutralize both acids and bases, maintaining a relatively neutral pH in biological systems. The amphoteric nature of water also enables it to participate in hydrolysis reactions, which are essential for many chemical and biological processes.
Ionization of Water
Water undergoes a process called autoionization, where a small number of water molecules ionize to form hydrogen ions (H+) and hydroxide ions (OH-). The concentration of these ions determines the pH of water, which is a measure of its acidity or alkalinity.
The ionization of water is fundamental to many chemical reactions and biological processes.
Biological Properties: Properties Of Water Crossword Puzzle
Water is essential for life on Earth, playing a crucial role in numerous biological processes.
Solvent for Living Organisms
Water is the primary solvent in living organisms, making up around 60-70% of their mass. It serves as a medium for transporting nutrients, waste products, and other molecules throughout the body. Water also participates in metabolic reactions and helps maintain cell shape and function.
Temperature Regulation
Water’s high specific heat capacity enables it to absorb and release large amounts of heat without significant temperature changes. This property helps regulate body temperature in living organisms, preventing overheating or excessive cooling.
Plant Growth and Photosynthesis
Water is essential for plant growth and photosynthesis. It provides the raw material for photosynthesis, where plants use sunlight to convert water and carbon dioxide into glucose and oxygen. Water also transports nutrients throughout the plant and helps maintain cell turgor, which is necessary for plant growth and support.
Environmental Properties
Water plays a critical role in the environment, influencing weather patterns, ecosystems, and human activities.
Water Cycle
Water undergoes a continuous cycle of evaporation, condensation, and precipitation, known as the water cycle. This cycle distributes water throughout the Earth’s surface and atmosphere, influencing weather patterns and the availability of water for living organisms.
Impact of Water Pollution
Water pollution can have devastating effects on ecosystems. Contaminants such as heavy metals, chemicals, and sewage can disrupt aquatic life, damage food chains, and harm human health. Water pollution can also lead to eutrophication, where excessive nutrients cause algal blooms that deplete oxygen levels and disrupt the balance of aquatic ecosystems.
Importance of Water Conservation
Water is a finite resource, and its conservation is crucial for sustainable development. Reducing water consumption, improving water efficiency, and protecting water sources are essential to ensure the availability of clean water for future generations.
Cultural and Historical Significance
Water has played a significant role in human culture and history, influencing art, literature, religion, and social structures.
Historical and Cultural Significance
Water has been a source of sustenance, transportation, and trade throughout history. Civilizations have flourished near water bodies, and water has shaped cultural practices and beliefs. Water has also been a source of inspiration for art, literature, and music, depicting its beauty, power, and mystery.
Role in Religious Rituals and Ceremonies
Water holds a sacred significance in many religions. It is used in baptisms, purification rituals, and other religious ceremonies. Water is often associated with life, renewal, and spiritual cleansing.
Impact on Art, Literature, and Music
Water has been a muse for artists, writers, and musicians throughout history. It has inspired paintings, sculptures, poems, songs, and other artistic expressions. Water’s beauty, power, and mystery have captivated the human imagination, leading to countless works of art that celebrate and explore its significance.
Essential FAQs
What is the unique density of water?
Water is one of the few substances that expand upon freezing, resulting in its unique density behavior. At 4°C, water reaches its maximum density, becoming denser than both warmer and colder water.
How does water’s high specific heat capacity affect the Earth’s climate?
Water’s high specific heat capacity allows it to absorb and release large amounts of heat without significant temperature changes. This property plays a crucial role in regulating the Earth’s climate by absorbing and distributing heat energy, preventing extreme temperature fluctuations.
What is the significance of water’s surface tension?
Water’s surface tension, caused by cohesive forces between water molecules, enables the formation of droplets, capillary action, and the ability of insects to walk on water. This property is essential for various biological processes and technological applications.