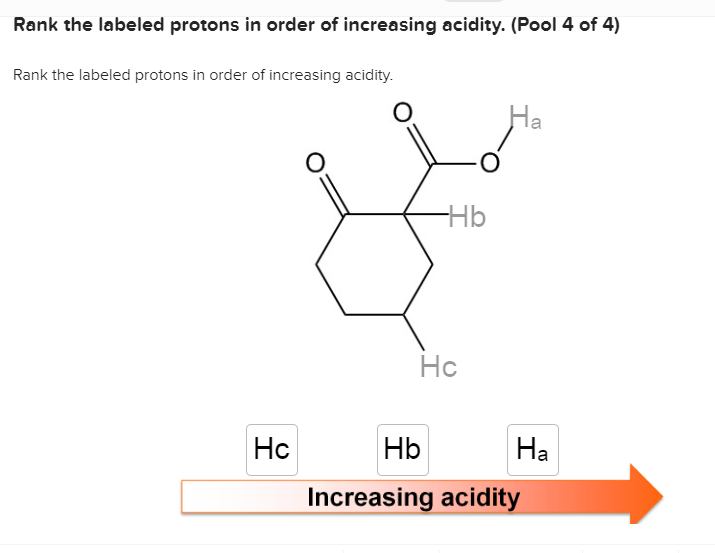
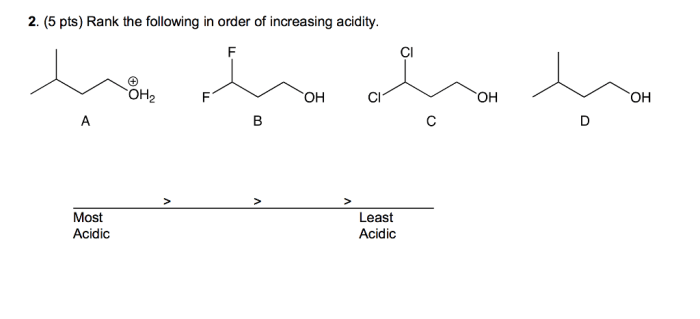
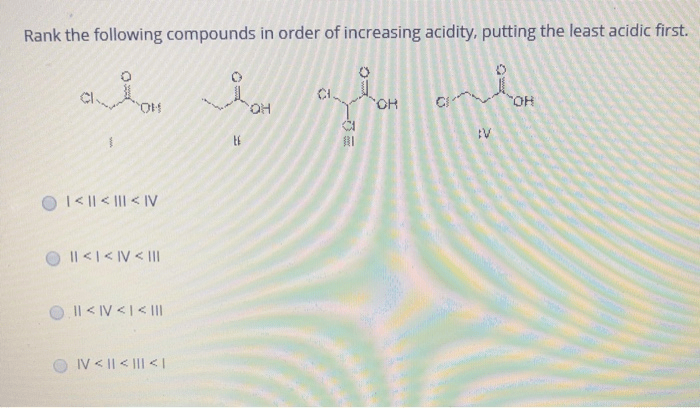
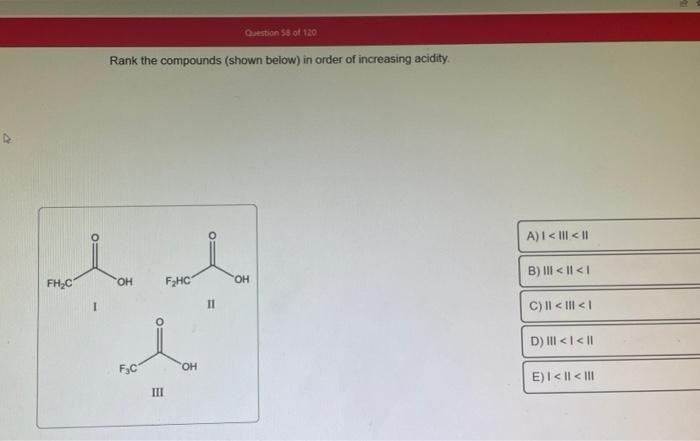
Rank the solutions below in order of increasing acidity. – Understanding acid strength is crucial in various fields. This guide explores the concept of acid strength, factors affecting it, and provides a step-by-step process to rank solutions in order of increasing acidity. By examining the relationship between pH and acidity, we gain insights into the implications of acidity in chemical reactions, industrial processes, and environmental impact.
Acid Strength Ranking
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. The pH scale is used to measure acidity, with a pH of 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity.
Several factors affect acid strength, including the strength of the acid-base bond, the size of the molecule, and the electronegativity of the atoms involved.
Solutions to Rank
| Solution 1 | Solution 2 | Solution 3 | Solution 4 |
|---|---|---|---|
| HCl | HNO3 | CH3COOH | NH4OH |
| 0.1 M | 0.1 M | 0.1 M | 0.1 M |
| 1 | 1 | 2.9 | 11 |
Ranking Process, Rank the solutions below in order of increasing acidity.
To rank the solutions in order of increasing acidity, we use their pH values. The lower the pH value, the higher the acidity.
Therefore, the solutions can be ranked as follows:
- Solution 1 (HCl, pH 1)
- Solution 2 (HNO3, pH 1)
- Solution 3 (CH 3COOH, pH 2.9)
- Solution 4 (NH 4OH, pH 11)
Implications of Acidity
Acidity has significant implications for various applications, including:
- Chemical reactions:Acidity can affect the rate and outcome of chemical reactions. For example, acids can catalyze reactions and dissolve certain substances.
- Industrial processes:Acidity is essential in many industrial processes, such as the production of fertilizers, plastics, and dyes.
- Environmental impact:Acidity can have a detrimental impact on the environment, contributing to water pollution, soil erosion, and damage to aquatic ecosystems.
FAQ Insights: Rank The Solutions Below In Order Of Increasing Acidity.
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity, and values above 7 indicate basicity.
How does pH relate to acidity?
pH is inversely proportional to acidity. The lower the pH, the higher the acidity, and vice versa. This is because pH measures the concentration of hydrogen ions (H+) in a solution, and higher H+ concentration indicates greater acidity.
What factors affect acid strength?
Acid strength is influenced by several factors, including the type of acid, its concentration, and the presence of other ions in the solution.